Are Your Herbs Spiked? Part 1
January 2, 1998
Are Your Herbs Spiked? Part 2
August 1, 2005Qualitative and Quantitative Near Infrared Spectroscopic Evaluation of the Raw Ingredients for Health Foods and Nutritionals
By Dr. Jeff Golini, All American Pharmaceutical, 2376 Main Street, Billings, MT, 59105
And Cynthia Kradjel, Don Muller, Arnold Eilert; Bran + Luebbe 1025 Busch Parkway, Buffalo Grove, IL 60089
Research by: Dr. Jeff Golini, All American Pharmaceutical, Bioceuticals Research & Development Analytical Division.
Presented at the Pittcon Conference, Orlando, Florida, March 1999
American consumers are increasingly supplementing or replacing prescription medicines with medicinal herbs, vitamins and minerals. An estimated $ 12 billion dollars was spent by Americans on natural supplements in 1997. In addition, the consumption of functional foods, nutraceutical drinks, and other types of dietary supplements are growing at a rapid pace.
Analytical requirements for this industry are characterized by the following key issues:
- The physical state of incoming raw materials, in-process formulations and final products can span a diverse range. For example, herbs can be formulated as capsules, tinctures, extracts, teas or added to common foods such as fruit drinks or potato chips. Samples may be liquids in the form of sublingual, tinctures, homeopathic, vitamins, cough and cold medicines, or special drinks; powders in the form of weight gain formulas, protein, carbohydrate or baby powders, cocoa mixes, drink mixes or spices; or capsules of vitamins, minerals, and herbs that may be clear, colored or printed on the exterior.
- Natural products have complex matrixes, which may contribute to their benefit for the consumer. The ability to measure the product without separating the constituents from their matrix is very important.
- Botanicals and phytochemicals and other supplements are regulated by the 1994
Dietary Supplement Health and Education Act. Industry leaders are striving to set high quality standards and implement Good manufacturing practice (GMP) principles in their manufacturing facilities.
- Speed and volume requirements make a fast testing method essential. For example, a shipment of incoming raw material may arrive in 25 drums, depending upon the volume. With traditional laboratory methods, it would be very time consuming to verify the identity and quality of every container of incoming raw material. A method that could be used in the receiving area and produce results in less than one minute would make the task achievable.
- For companies that sell standardized herbals, there is an increasing challenge to develop analytical methods to accurately quantify the assay. Potency of herbs can vary from batch to batch so in order to use the NF (National Formulary) designation on the label, standardization testing must be performed.
- Purity testing is becoming more important as demand for botanicals, herbals and natural products grow, supply issues put pressure on raw material suppliers. Manufacturers must look for the presence of spiked or doped material. Similar raw materials can often fool traditional tests, so manufacturers order a $2000/kg raw material and may receive a $200/kg similar raw material.
- A new federal rule published September 23, 1997 dictates changes to labeling and includes a box of “supplement facts” similar to the “nutrition facts” label found on food products. Standardized products will state the amount of active ingredient in each dose. Herbal products will be identified by the common or usual name the labels and include which part of the plant is used, such as the root, stem or leaf. These new requirements challenge traditional testing methods.
- Products tend to move quickly into and out of the marketplace. Method development must be flexible, rapid and responsive.
An analytical technology has been identified and implemented to address the eight key issues effectively. This technology is Near Infrared Analysis. Heavily used in the pharmaceutical industry, the technology is based the interaction of light with the material to be analyzed. Sophisticated mathematical algorithms are used to store, interpret and present results.
Near Infrared Technology has been successfully implemented at All American Pharmaceutical (the pioneers in NIR for the Nutrition Industry) using the Bran + Luebbe InfraProver for routine testing of identity and quality of all inbound raw materials as ingredients for health foods and nutritionally beneficial formulations. Every container entering the facility is tested to guarantee that identity, purity, safety, and potency standards are met. The containers are sampled and then quarantined until completely approved. Once testing is completed and the material is approved, each ingredient is issued a Certificate of Analysis which corresponds to each Lot number. Their products range from a wide variety of health food ingredients, herbals and nutritional supplements. The products range in physical form from solids, liquids, gels and tablets. Qualitative methods are used for identity and quality testing and quantitative methods are used for in- process optimization and for generation data for Certificate of Analysis (COA).
The Bran + Luebbe InfraProver is a polarization interferometer Fourier transform NIR instrument based on principles of HPCS (High Performance Crystal Spectroscopy). The system uses a moving crystal wedge to obtain FT Interferograms for the materials being tested, resulting in a substantial reduction in sensitivity to vibration compared to traditional lab grating and FT instruments. This type of optical system enables the instrument to be placed on a cart and moved to the receiving area or warehouse area, without compromising the precision of the results.
The design of the instrument allows All American Pharmaceutical to present a wide variety of sample forms to the system via software control without changing the hardware. Two options are available for solids. A 2-meter fiber optic probe enables “probing” of containers in the reflectance mode. Materials can be scanned directly in the containers or through plastic packaging material. Alternatively, the Solid Presentation Accessory (SPA) permits spectroscopic interrogation through the base of a vial when the vial is placed on the optical window of the SPA. Tablets or capsules can also be analyzed with this accessory. Liquids were scanned in the transmission cell, a “built-in” cuvette chamber located in the center of the InfraProver.
Long term stability of the instrument is achieved via internal laser referencing for all wavelengths ensuring wavelength accuracy and reproducibility.
Near Infrared technology scans the product in its entirety. Mathematical algorithms then separate the chemical or physical properties of interest from the rest of the matrix. Samples are therefore analyzed “as is” with no sample preparation.
The InfraProver was originally developed for the pharmaceutical industry so it was designed to meet GLP, GMP principles. Many pharmaceutical companies have validated the instrument for their applications.
Once a calibration has been developed, identity, quality and quantity can be analyzed in less than 60 seconds. Operator level functions are present for routine use.
Assay methods have been developed enabling All American Pharmaceutical to meet new label requirements. Purity can be determined, giving manufacturing the information that incoming raw materials have been spiked or doped. In some cases, NIR has shown that similar materials were substituted or co-mingled with incoming raw materials.
Method development can be turned around to adapt the technology to new products or new formulations.
Several examples of All American Pharmaceutical’s research along with their technology are described below.
Library Identification Method
Identification is performed using a Library model. Several batches of each raw material are placed in glass vials, which are placed on the optical window of the Sample Presentation Accessory (SPA). The InfraProver scans the raw materials through the glass vial. Mathematical modeling is used to determine acceptable radii and residual for each material in factor space. When an unknown sample is scanned, the Mahalanobis distance radii and spectral residual are evaluated in relation to those used to develop the library
model. If the radii and residual are within the established criteria for a material, the InfraProver will confirm that the identity is ok. If the radii and residual are not within the established criteria, the InfraProver will inform the user that the identity is not ok, will print out a list of nearest materials to the one scanned.
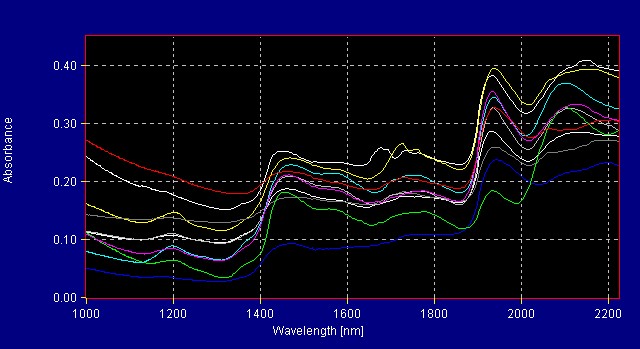
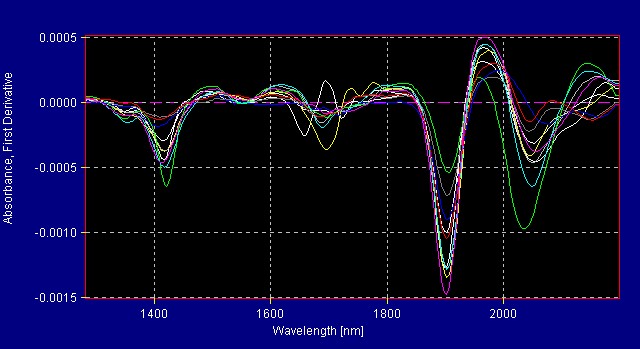
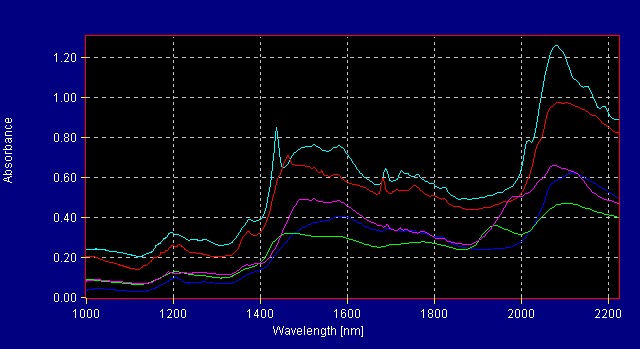
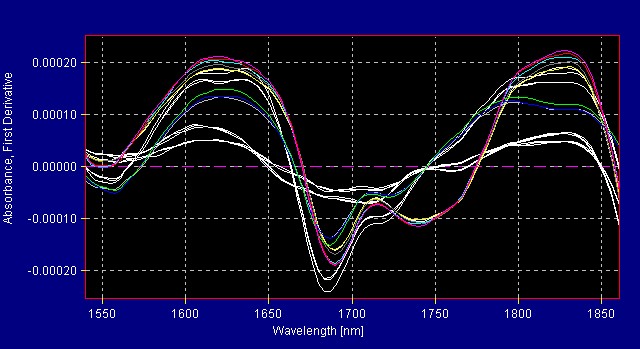
All American Pharmaceutical analyzes more than 300 herbals with the InfraProver. The herbals are grouped into several library models. Figure 1 shows the reference spectra for herbals in one of the herbal libraries. Mathematical data treatment such as first derivative makes the differences more noticeable to the eye. The mathematical algorithms view the data in multivariate space and are able to resolve very small differences that are not obvious to the human eye. Figure 2 shows the same materials in Figure 1 now graphed using first derivative data treatment.
Figure 1
Figure 2
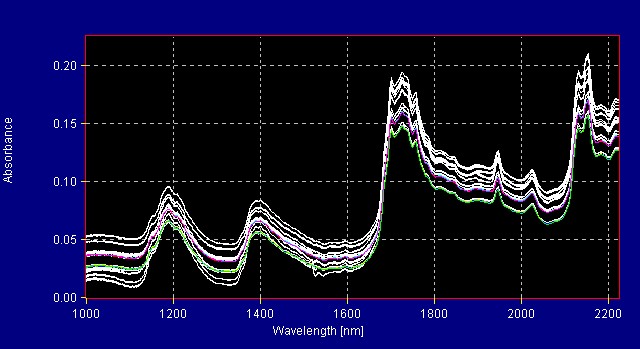
Other libraries in use at All American Pharmaceutical include: Amino acids (Figure 3), Carbohydrates (Figure 4), Vitamins (Figure 5), and Sugars (Figure 6). Miscellaneous Materials containing a variety of molecular species, which were easy to distinguish, were grouped into another model. This testing program assures proper ingredient identification and prevents formulation with incorrect or defective ingredients.
Figure 3: Amino Acids
Figure 4: Carbohydrates. This model could distinguish between instant maltodextrin and 100-mesh maltodextrin.
Figure 5: Vitamins
Figure 6: Sugars
Cluster Models
Qualitative cluster Soft Independent Modeling Cluster Analysis (SIMCA) models were particularly effective in distinguishing similar materials. This type of mathematical algorithm is used when library models cannot separate close substances. All American Pharmaceutical has been able to use cluster model to identify which supplier sourced the material, which has been especially important for vitamins.
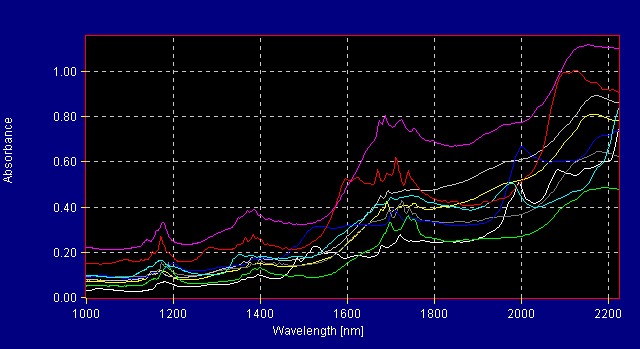
Another interesting example of this type of model is to separate between raw burdock root powder and (a 4:1) burdock root extract.
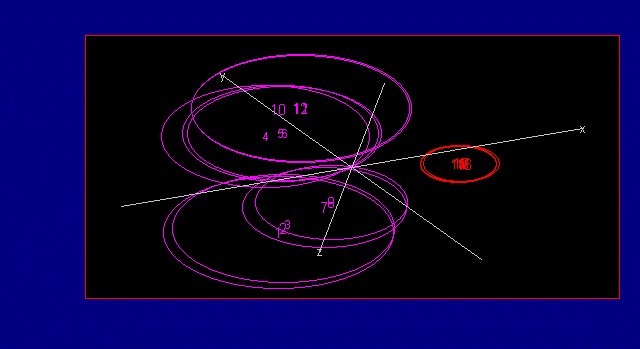
The extraction process is used to concentrate the active ingredients of a raw material. Dried extract (powder form) is desired for use in manufacture of nutraceutical products. Figure 7 (below) shows the difference between the spectra of the raw burdock root powder and the burdock root extract. Figure 8 (below) shows a graphical representation of the different groups (raw burdock root and burdock root extract) known as a 3-factor cluster plot.
Figure 7: Raw Burdock Root and Burdock Root Extract
Figure 8: Three Factor Cluster Plot shows the two groups
Quantitative Analysis
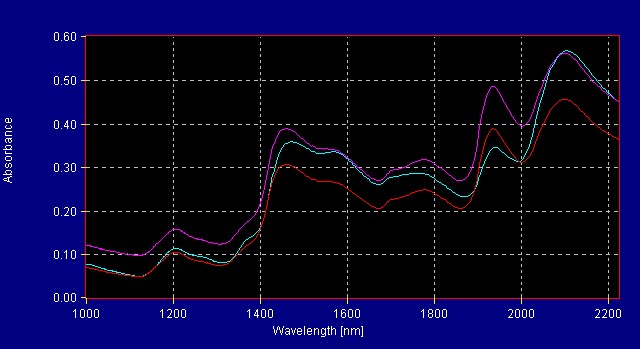
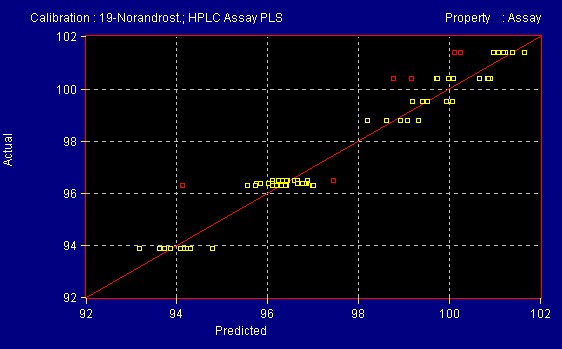
A quantitative method was developed at All American Pharmaceutical for 19- Norandrosteine Assay. A population of teaching samples usually 20 samples with varying references values are scanned into the InfraProver. Partial Least Squares, Multiple Linear Regression and Principal Component Analysis mathematical algorithms are used to evaluate the data and generate a correlation of the spectra to the reference method. This results in a formula that can be used to determine the assay of unknown materials of the same product type as those used to teach the system. Figure 9 shows spectra of varying concentration of Assay of 19-Norandrosteine. The reference method was an HPLC method (Figures 10 and 10a). The InfraProver was able to predict unknown 19-Norandrosteine Assay in the range of 92 – 102 Assay.
Figure 9
Figure 10
Figure 10a
Conclusion
All American Pharmaceutical has successfully implemented Near Infrared methods using the Bran + Luebbe InfraProver. This approach offers an elegant solution to the key issues facing analytical method developments for dietary supplements and nutraceuticals.
All American Pharmaceutical has become the pioneer of NIR in the Nutraceutical Industry. They have developed 100’s of validated testing method’s, which provide all their customer’s with the quality assurance they have come to rely on and expect from All American.
All American Pharmaceutical has developed and adjusted NIR methods when necessary to adapt to the changing needs of their industry by providing rapid identity and quality confirmation. NIR enables qualitative evaluation of raw materials to verify the quality of incoming raw materials (keeping vendors honest) and ensure product consistency. Quantitative analysis of active ingredients and other important properties of both raw materials and final products can be determined effectively.